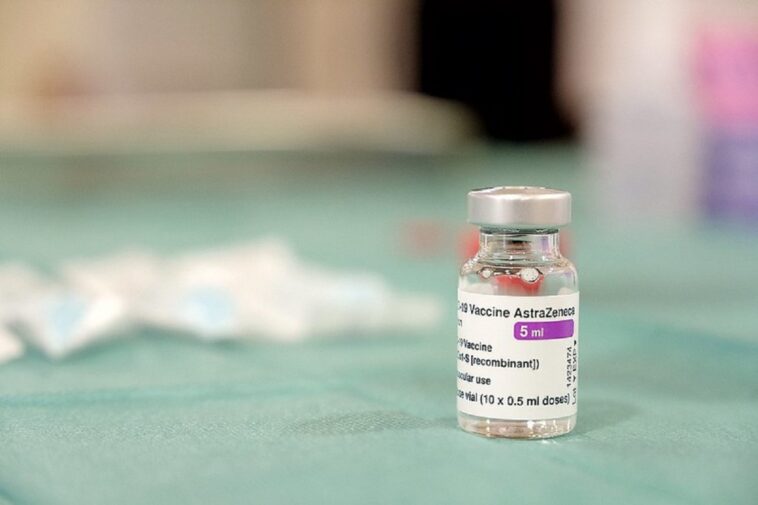
Norway has followed the suit, which temporarily emphasized the use of Covid-19 vaccine from AstraZeneca.
Denmark exposes the use of Astrazeneca Covid-19 vaccine
Denmark has been exposed to the use of the Astrazeneca Covid-19 vaccine after some people after some people who received the JAB later developed blood clots. The country’s health office said that the vaccine does not give any evidence that the vaccine is behind the clots, but the use of use as a precautionary measure, as it further examines the matter.
“Currently, it can not be closed if there is a connection between the vaccine and the blood clots,” said Denmark’s health authority in a statement, indicating that his decision to take the use of the JAB, “based on a precautionary principle” and that one Person, who developed a blood clot after vaccination, had died.
In a release, the FOX News was provided, the European Medicines Agency said that it was aware that the country installed the use of the Jabs, but noted that it can be administered as the examination continues to the blood clot. “There is no indication that the vaccine has caused these conditions that are not listed as side effects with this vaccine,” said the controller. “The position of the EMA Security Committee is that the benefits of the vaccine continue to predominate its risks, and the vaccine can still be managed while the investigation of cases is running thromboembolic events.”
Coronavirus declared a year ago global pandemic: “We endure together”
The EMA in his release also found that the number of thromboembolic events in vaccinated people “is not higher than the number in the general population.” “By March 10, 2021, 30 cases of thromboembolic events were reported in nearly 5 million people who were vaccinated with the Covid 19 vaccine AstraZeneca in the European Economic Area,” said it.
Some experts also pointed out that the millions of AstraZeneca vaccine recordings that were managed elsewhere, including in the United Kingdom, did not give any cases of the vaccine that causes blood clots or related problems. Others have pointed out that pausing vaccinations at a time when the world is still struggling to control the Roman Coronavirus, as vaccination of vaccinations are more at risk for the contract and the possible die of Covid-19 could bypass.
“We are extremely encouraged that the real effectiveness data derived from Israel, confirming the high effectiveness that demonstrated in our clinical phase 3 and shows the significant impact of the vaccine to serve serious illnesses and deaths due to Covid-19″ Luis Jodar ” To prevent Ph.D., Senior Vice President and Chief Medical Officer of Pfizer vaccines, said in the press release. “The results suggest that the vaccine can also provide protection against asymptomatic SARS COV-2 infections, are particularly meaningful because we interrupt the spread of the virus around the world. Overall, these data are critical to understand the role of vaccination in combating the pandemic and ensure other countries that deal with this devastating disease that has been concerned for more than a year of the world. ”
The controller of the British drug controller was implemented by notizing that the reported blood clot was caused by the Astrazeneca vaccine. Biden set to accept nation at the anniversary of the Covid 19 pandemic. “People should still go and get their Covid 19 vaccine when it comes to doing this,” said it.
Even after the Danish announcement, Norway decided to follow the suit and temporarily suspend the Anglo-Swedish Company vaccine developed by the University of Oxford, and also said that there are no proven links. In addition, the pharmaceutical agency Italy ordered a precautionary ban on a certain batch of the AstraZeneca vaccine on Thursday, after he had said “serious undesirable events. ”
On Wednesday, the EMA said that Austria was the use of a batch of Covid-19 vaccine manufactured by AstraZeneca, after a person had been diagnosed with several blood clots had been assumed. The latter is recovering now.
Together with the Israel Ministry of Health, Pfizer and Biontech announced “real-world evidence”, they say that their Covid-19 vaccine “dramatically” reduces the rates of the coronavirus in vaccated individuals. In a press release posted on Thursday, companies said that two weeks after the second dose, the vaccine after the second dose, was at least 97% in preventing symptomatic diseases, severe/critical illness, and death.
The analysis draws on data collected from 17 January to March 6, a time when the Pfizer and BionTech two-dose jab was the only Covid-19 vaccine approved in Israel and as the B. 1.1.7 variant, which is assumed by a higher transferability, was the dominant burden on the country. In addition to the very effective of symptomatic disease, the vaccine was found as 94% to prevent asymptomatic SARS COV 2 infection according to the press release.
Two more reports of similar problems were received for this batch for March 9, the EMA said, although there is no indication that the problems were caused by the vaccine.
The EMA said that the 1-million dose batch in question was delivered in 17 EU countries.
The Germany’s Paul Ehrlich Institute, the vaccine issues supervised, said it was in contact with Danish authorities and the EMA in the question, but that no doses were from the batch used in Austria in the German market.
Denmark’s suspension of the AstraZeneca vaccine will take at least two weeks, said the agency, said the agency and attached it to an evaluation of the EU-medical regulator.
A speaker for AstraZeneca came to the request of Fox News not immediately back to comment.
The vaccine was found as at least 97% to prevent symptomatic and heavy COVID-19
The results follow a similar study published last month from the Clalit Research Institute and several US universities that reduced the vaccine symptomatic COVID 19 cases by 94%. The peer-review study showed consistency with the 95% vaccine efficiency, which was reported first in the clinical trials.
- Facebook Messenger